
It has also been shown that both embryonic lethality and increased levels of CNS neuronal apoptosis in mice with deficiency in Lig4, Xrcc4, Xlf and Paxx, or Xlf and Dna-pkcs is p53-dependent. However, combined inactivation of Xlf and Paxx, as well as Xlf and Mri, results in late embryonic lethality in mice, presenting a challenge to the study of B and T lymphocyte development in vivo. The mild phenotype observed in mice lacking XLF could be explained by functional redundancy between XLF and multiple DNA repair factors, including Ataxia telangiectasia mutated (ATM), histone H2AX, Mediator of DNA Damage Checkpoint 1 (MDC1), p53-binding protein 1 (53BP1), RAG2, DNA-PKcs, PAXX and MRI.

Inactivation of Xlf ( Cernunnos) only results in modest immunodeficiency in mice, while mice lacking Paxx or Mri display no overt phenotype. Deletion of both alleles of Xrcc4 or Lig4 results in late embryonic lethality in mice, which correlates with increased apoptosis in the central nervous system (CNS). Inactivation of Ku70, Ku80, Dna-pkcs or Artemis results in severe combined immunodeficiency (SCID) characterized by lack of mature B and T lymphocytes. The NHEJ complex is stabilized by a paralogue of XRCC4 and XLF (PAXX) and a modulator of retroviral infection (MRI/CYREN). Finally, DNA ligase IV (LIG4), X-ray repair cross-complementing protein 4 (XRCC4) and XRCC4-like factor (XLF) mediate DNA end ligation. Subsequently, the nuclease Artemis is recruited to the DSB sites to process DNA hairpins and overhangs.

Ku, together with DNA-dependent protein kinase, catalytic subunit (DNA-PKcs), forms the DNA-PK holoenzyme.

NHEJ is initiated when Ku70 and Ku80 (Ku) are recruited to the DSB sites. NHEJ is required for lymphocyte development in particular, to repair DSBs induced by the recombination activating genes (RAG) 1 and 2 in developing B and T lymphocytes, and by activation-induced cytidine deaminase (AID) in mature B cells. Non-homologous end-joining (NHEJ) is a DNA repair pathway that recognizes, processes and ligates DNA double-stranded breaks (DSB) throughout the cell cycle. Moreover, Mri genetically interacts with Dna-pkcs and Paxx. Therefore, we conclude that XLF is functionally redundant with MRI and PAXX during lymphocyte development in vivo. We also report that combined inactivation of Mri/Paxx results in live-born mice with modest phenotype, and combined inactivation of Mri/Dna-pkcs results in embryonic lethality. Furthermore, Xlf -/-Mri -/-Trp53 +/- and Xlf -/-Paxx -/-Trp53 +/- mice possess reduced body weight, severely reduced mature lymphocyte counts, and accumulation of progenitor B cells. We demonstrate that deletion of Trp53 rescues embryonic lethality in mice with combined deficiencies of Xlf and Mri.
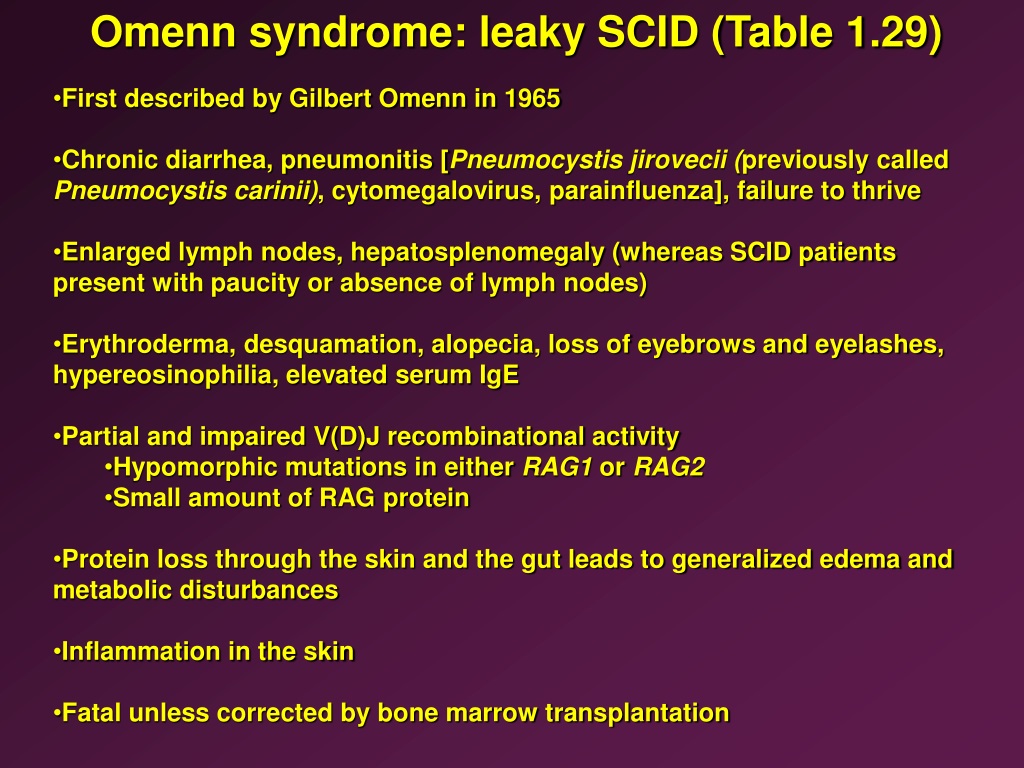
Here, we describe three new mouse models. Inactivation of Mri, Paxx or Xlf in mice results in normal or mild phenotype, while combined inactivation of Xlf/Mri, Xlf/Paxx, or Xlf/ Dna-pkcs leads to late embryonic lethality. Mutations in several known NHEJ genes result in severe combined immunodeficiency (SCID). During NHEJ, Ku70 and Ku80 form a heterodimer that recognizes DSBs and promotes recruitment and function of downstream factors PAXX, MRI, DNA-PKcs, Artemis, XLF, XRCC4, and LIG4. The NHEJ pathway is necessary for V(D)J recombination in developing B and T lymphocytes. Non-homologous end-joining (NHEJ) is a DNA repair pathway required to detect, process, and ligate DNA double-stranded breaks (DSBs) throughout the cell cycle.


 0 kommentar(er)
0 kommentar(er)
